Ultra-fast, on-site diagnostics of coronavirus as a lifesaver
Diagnostics
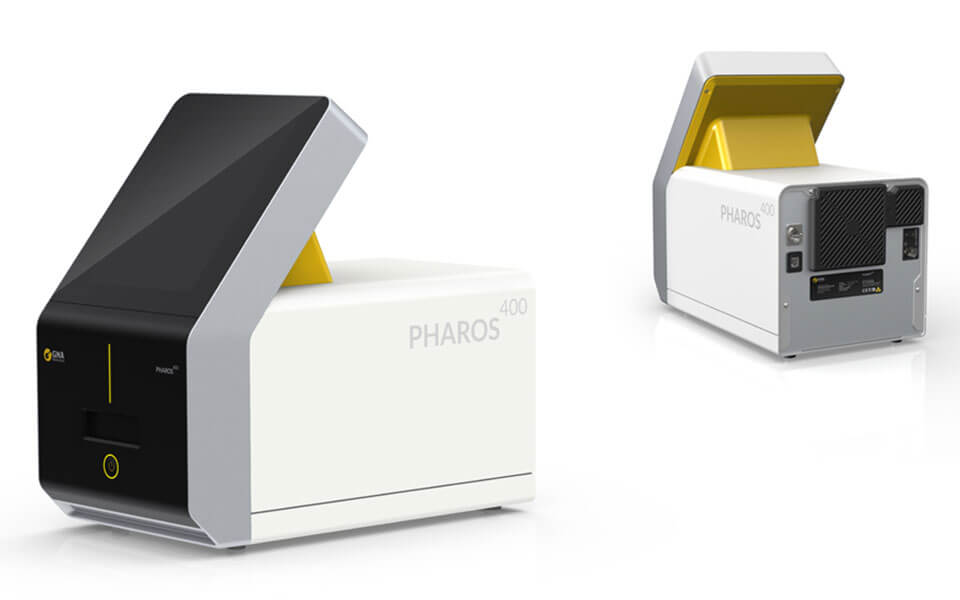
GNA Biosolutions GmbH (SHS IV Fund)
Test, test and test again! Diagnostics play a critical role in combating the current coronavirus pandemic (SARS-CoV-2). The quick and comprehensive identification of infected persons is a crucial contribution to the detection and critical disruption of chains of infection. With its ultra-fast PCA (pulse-controlled amplification) technology, GNA, headquartered in Martinsried near Munich, has developed a molecular diagnostic platform that will also help combat the coronavirus pandemic beginning June 2020 (assuming final test results are positive). For its innovative development, GNA was presented with the coveted “Disruptive Technology Award” at AACC, the largest conference for diagnostics. GNA’s technology has key advantages over the testing systems currently offered by other providers:
- The test can be carried out directly on-site (point of care – PoC)
- Test results are quickly available (after approx. 15 min.)
- Except for the sampling itself, the test is fully automated
- The test does not require specially trained staff to carry out
- The test device is mobile and can be used even under difficult climatic conditions
The fact that patient samples no longer have to be sent away to a laboratory for testing, and that results are available quickly, is a great advantage, particularly when the goal is to gradually restart the economy and public life. Thanks to the GNA test, infected individuals can be identified quickly, and a new wave of infection can potentially be avoided through quarantining.
Further information: www.gna-bio.com